Contract Manufacturing Medical Devices
VTL specializes in being a medical device contract manufacturer, offering comprehensive solutions from engineering services to product development and fabrication. We produce several medical devices and products that are either sewn, sealed, or both.
Custom contract manufacturing of medical devices includes vacuum lock cushions for positioning patients for imaging exams, compression garments, and environmental control products to accelerate the healing process.
Our Medical Device Solutions Include:
- Engineering services
- Product development
- Fabrication of resources
- Integration support
Types of Products and Contract Manufacturing Medical Devices:
- Medical Inflatable Bladders
- Hospital Headpieces for Trauma Patients
- Blood Pressure Cuffs
- Compression Garments
- Containment Walls for Isolation Rooms
- Vac-lock Cushions and other Medical Positioning Devices
- Custom Medical Devices
- Custom Sewn Medical Products
- Mattress Overlays
Our medical device contract manufacturer expertise ensures that every product meets the highest standards of quality and reliability. VTL can assist with material selection, product engineering services, quality assurance testing, tooling, RF sealing, industrial sewing, and product assembly.
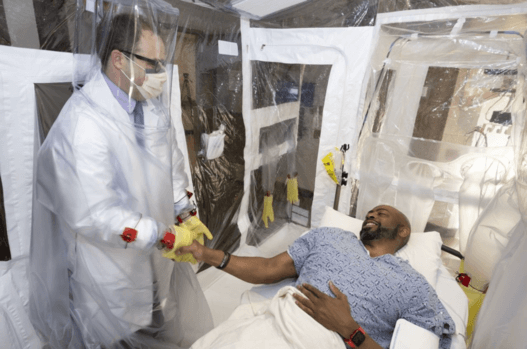
Isolation Rooms
One of our standout contract-manufactured medical devices, VTL helped create temporary, low-cost, in-room, negative pressure tents that make it easier for hospitals to scale their response to infectious disease events like the COVID-19 pandemic of 2020.
- Isolation rooms like this have a clear-view PVC or TPU canopy with a high-contrast floor and RF-welded seams. Double lap hook & loop allows replacement of glove, conduit panel, and hug suit components.
- External steel frame and disposable tent with integrated glove walls and HugSuit for full patient access with significantly less need for PPE.
- Air handling is included and meets the 15 air exchanges per hour (ACH) CDC guidelines for surgical procedure and delivery rooms. The .3-micron HEPA and MERV intake filters are welded directly to the tent canopy, greatly mitigating the risk associated with user error and improving safety during the disposal process.

Vac-Lok Cushions
VTL also manufactures vacuum lock cushions used to stabilize patients in preparation for medical procedures such as MRIs and CAT Scans. Cushions form to the patient’s body contour, maintaining the patient’s position over repeated visits for improved tracking of the patient’s progress.
The contract-manufactured medical device is filled with styrofoam beads and then sealed shut. A valve allows for a vacuum to be pulled on the product before it is finally sealed, tested, and packed for shipment.

Why Choose VTL For Your Contract Manufacturing Medical Devices?
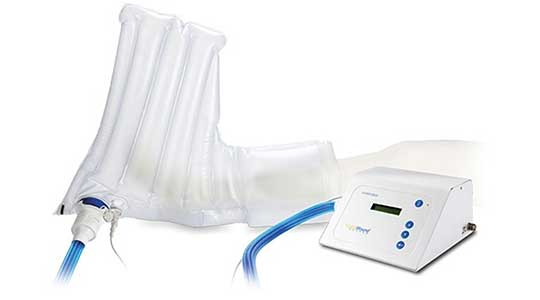
VTL produces many contract-manufactured medical devices and products for the highly demanding medical industry. The inflatable boot (pictured here) enables environmentally controlled therapy of open wounds and other conditions to accelerate the healing process. Its production posed a challenge as it required a complex combination of sequenced operations and advanced tooling.
VTL engineers came through and created a manufacturing method and tooling specifically for this customer. The application was a breakthrough in the treatment of slow-to-heal extremities. The customer and its end users were thrilled with the outcome.
Choosing VTL for your contract manufacturing medical devices and needs means partnering with a company committed to excellence in every aspect of production, from material selection to final assembly.
Contact us today to discuss what contract manufacturing medical devices you need.
Certifications
Vinyl Technology, LLC complies with standards required by the US government and Department of Defense that include the National Institute of Standards and Technology (NIST), The International Traffic in Arms Regulations (ITAR), Defense Federal Acquisition Regulation Supplement (DFARS) to the Federal Acquisition Regulation (FAR), STANAG 4671 and MIL-STD-6396. VTL can also fabricate products that comply with the Berry Amendment when required.
VTL’s Quality System is currently certified AS9100D and ISO9001:2015. Since 2014, VTL has implemented LEAN Manufacturing practices, Six Sigma, and Kaizen methodologies to improve operations. Vinyl Technology is an FDA registered manufacturer for medical products.
Our certifications underscore our commitment to the highest medical device contract manufacturing standards, ensuring compliance and quality for our partners.

Medical Device Contract Manufacturing FAQ
What does it mean to be FDA Registered?
Licensed owners or operators of medical facilities involved in the production or distribution of medical devices are required to register annually with the FDA if the intended uses of those medical devices are for the United States. This does not signify FDA approval of products manufactured through VTL.
Where are medical devices manufactured?
VTL manufactures medical devices at our facilities in Monrovia, California.
What is contract manufacturing for medical devices?
Contract manufacturing is when specialized companies like Vinyl Technology handle the production of medical devices. This outsourcing gives original equipment manufacturers (OEMs) the freedom to focus on research and development, while production stays efficient and compliant with industry regulations.
What types of medical devices does Vinyl Technology manufacture?
We offer contract-manufacturing medical devices, such as inflatable mattresses, chair cushions, inflatable medical bladders, blood pressure cuffs, custom-sewn medical products, trauma patient headpieces, compression garments, and containment walls for isolation rooms.
Can Vinyl Technology assist with the design and development of custom medical devices?
Yes, our engineering team works closely with you to design and develop medical devices created specifically for your needs. From initial concept to final production, we make sure your custom product meets your specifications and all required regulations.